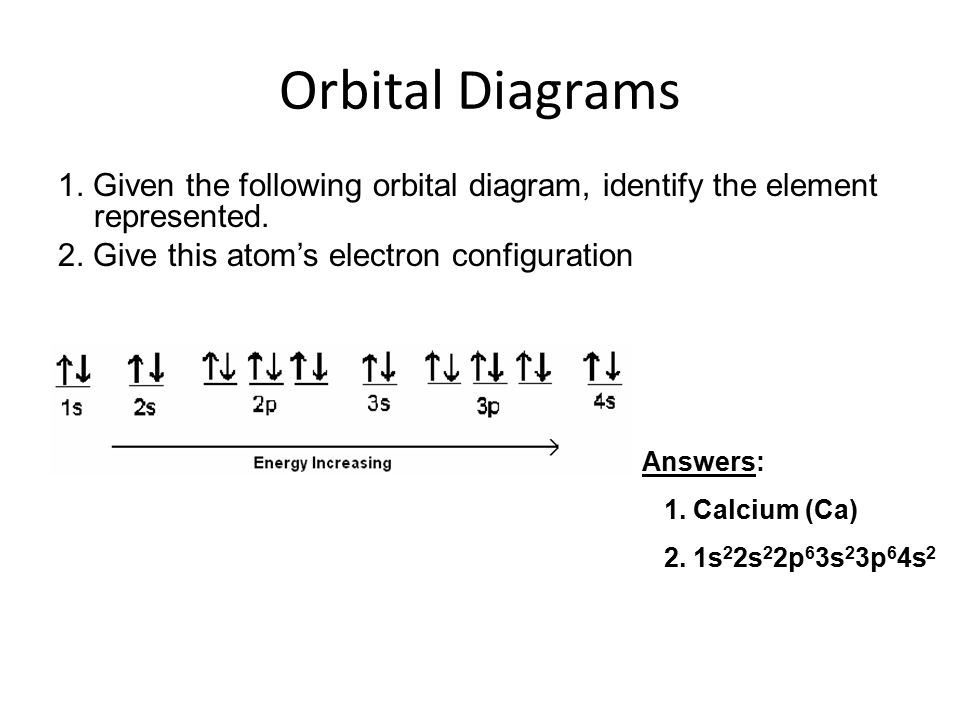
orbital diagram for ca
Carbon Electron Configuration is an element of the periodic table and its atomic number is 6. The orbital diagram will be filled in the same order as described by the Aufbau principle.
Electrons Gps Ppt Video Online Download
To write the orbital diagram for the Calcium atom Ca first we need to write the electron configuration for just Ca.
. 1s 2s. Electron configurations have the format. Orbital diagrams Orbital box diagrams of all elements are mentioned in the.
83 6 ratings Calcium has atomic number of 20 so it has total of 20 electrons which should. Part B Enter an orbital diagram. The orbitals are p x p y and p z and each orbital can have a maximum of two electrons.
Ad Over 27000 video lessons and other resources youre guaranteed to find what you need. What is the orbital diagram for Calcium Ca. Orbital diagrams Orbital box diagrams of all elements are mentioned in the chart given below.
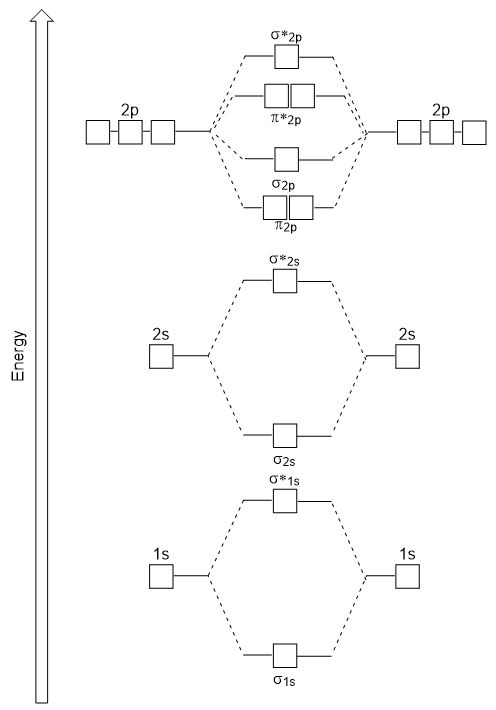
In writing the electron configuration for Calcium the first two electrons will go in the 1s orbital. July 16 2022 by Sneha Leave a Comment. The head-to-head overlap giving σ molecular orbitals results in greater overlap making its bonding molecular orbital the most stable and lowest energy while the σ antibonding is least.
How To Write The Orbital Diagram For Calcium Ca 0215 min 320 kbps 309 MB Downlod Now. Cr 2e Cr 2. View the full answer.
Therefore the gallium full electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 1. How many users might wonder what exactly will be the problem with that. No because due to the Auf-bau rule e- enters the 4s sub-shell of Ca instead of going in 3d sub-shell so Ca does not have any vacant d-orbital.
Are you searching for Calcium Electron Configuration Ca with an Orbital Diagram. Here the electron configuration of chromium ion Cr 2 is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 4. Since 1s can only hold two electrons the next 2 electrons for Calcium go in the 2s orbital.
The first number is the principal quantum number n and the letter represents the value of l angular momentum. So the remaining one electron enters the 4p orbital. 1s 2 2s 2 2p 6.
The Pauli Exclusion Principle - This says that only two electrons can stay in a single orbital. Every person should learn about the chemical. The chromium atom donates an electron in 4s orbital and two electrons in 3d orbital.
Create the orbital diagram for sodium. What is the electronic. To do that we need to find the number o.
Orbital Diagrams And Electron Configuration - Basic Introduction - Chemistry Practice. Part B Enter an orbital diagram. This problem has been solved.
The order in which the orbitals are filled with electrons from lower energy to higher energy is. Therefore the electron configuration of calcium Ca in excited state will be 1s 2 2s 2 2p 6 3s. Ad Save Time Adding Watermark in Online PDF.
View the full answer. Hunds rule - This states that electrons enter different orbitals in the same sublevel before.

Electron Configuration For Carbon C
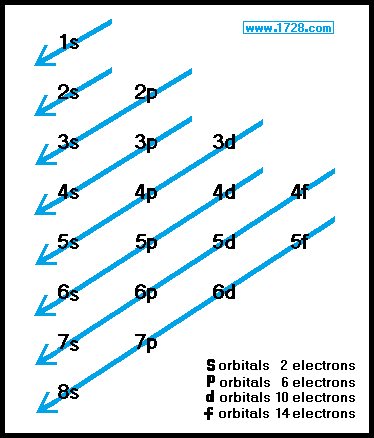
Orbitals And Electron Configuration
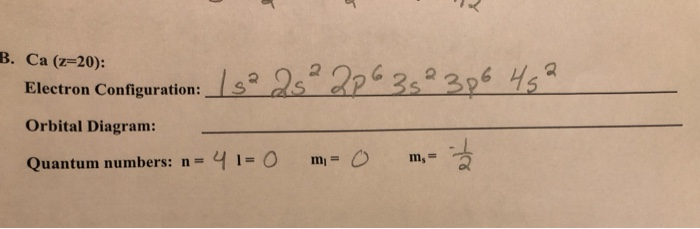
Solved B Ca Z 20 Electron Configuration Electron Chegg Com
What Is The Electron Configuration Of Calcium Ion Quora
Electron Configuration Quiz Learning Lab

Electron Configurations The Periodic Table

Orbital Configurations Ck 12 Foundation
Ch 1 Orbital Fillling Electron Configurations

Draw Orbit Structure Diagram Of Calcium Oxide Cao Chemistry Shaalaa Com

Molecular Orbitals Introductory Chemistry 1st Canadian Edition

Draw The Orbital Diagram Ca 2 Ion And State The Number Of Three Fundamental Particles Present In It

How To Draw The Bohr Rutherford Diagram For Calcium Youtube
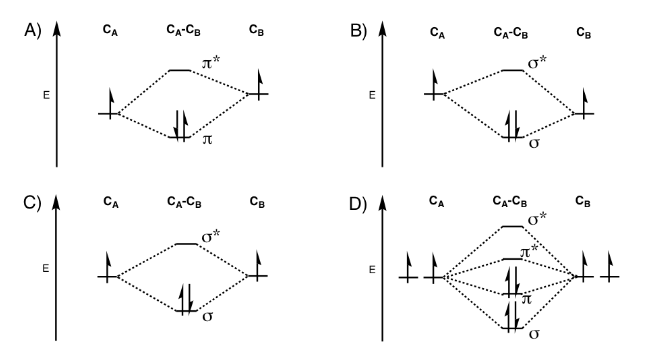
Solved Which Image Shows The Molecular Orbital Diagram For Chegg Com

Orbital Diagram Calculator Get Instant Answer
How To Write The Orbital Diagram For Calcium Ca Youtube
Electron Configuration Quiz Learning Lab

High School Chemistry Orbital Configurations Wikibooks Open Books For An Open World